
Broussard: 'Progeny from the vaccinated hens had 72% less chance of mortality'
 "And once you've
removed the protective effects of the subtherapeutic antibiotics, necrotic enteritis plays a bigger role."
"And once you've
removed the protective effects of the subtherapeutic antibiotics, necrotic enteritis plays a bigger role."
DR. CHARLIE BROUSSARD
The world's first vaccine for managing
necrotic enteritis in broilers is designed to
combat a toxin produced by Clostridium
perfringens type A—the toxin that is associated
with development of the disease.
Officially known as Clostridium perfringens
type A toxoid, the conditionally approved
vaccine employs an inactivated fragment of
the toxin, called a toxoid, to elicit immunity
to the toxin. The hens then pass on that
immunity on to their progeny.
The vaccine, developed by Schering-Plough Animal Health, got high marks in
laboratory studies and in limited field trials.
But how would this NE toxoid vaccine
perform in real-world conditions?
To find that out, Schering-Plough put
together a series of trials, vaccinating 1.5 million hens and generating data on more
than 21 million of their progeny.
At the Orlando symposium, Dr. Charlie
Broussard, worldwide technical service
veterinarian for the company's poultry
business unit, reported on trials aimed at
evaluating the NE toxoid's efficacy in broilers
that received no antibiotics.
Antibiotic-free birds face increased risk of NE
Broussard noted that this arm of the study
was especially important because more
and more broiler producers are bending to
consumer and regulatory pressures by
pulling antibiotics and growth promoters
from their birds' rations.
"And once you've removed the protective
effects of the subtherapeutic antibiotics,
necrotic enteritis plays a bigger role,"
he said.
Broussard also stressed that while
producers are well aware of how many
birds NE can kill, they tend to be less
familiar with the devastating effects
that subclinical disease can have on the
performance of their flocks. In one recent
survey, U.S. veterinarians who were polled
said they believe losses from subclinical
NE may total as much as 5 cents per bird.
"So there's a lot of performance loss going
on in addition to what most people think of
as necrotic enteritis—dead birds,"
Broussard said.
The company chosen for the site of the
antibiotic-free trial had a history of NE,
especially during cold and rainy weather.
The trial began in February 2005; about
80,000 pullets were vaccinated via
intramuscular injection and were given
a booster vaccination 8 weeks later.
The vaccinated pullets were then shipped
to 7 breeder farms. "The pullets were
vaccinated over time," Broussard
explained, "so we had breeder ages all
the way from young to the old in the trial."
Eggs were collected 4 times each day at
the farms and the chicks hatched.
Before he discussed the results of the trial
on the progeny from the vaccinated hens, Broussard emphasized an important point.
He said that for evaluation purposes,
progeny flocks in the trials were divided
into only two categories:
- Flocks in which all the birds
came from vaccinated hens
- Flocks in which less than all the
birds came from vaccinated hens
Broussard emphasized an important point.
In some cases, he pointed out, the flocks
categorized as nonvaccinated progeny
contained a sizable number of birds whose
hen parent had been vaccinated. In total,
the trial included about 1.3 million birds in
the all-vaccinated group and about 4.6
million in the standard group. Bird size of
the progeny ranged from about 4.5 to 5
pounds (2.04 to 2.27 kg).
One other caveat: During the trial, when
any flock exhibited NE-related mortality
at a rate of 4 birds per 1,000 or higher,
they were treated with the antibiotic
sulfadimathoxine. "So you need to factor
in that any time mortality really started
going out of whack for these birds in the
standard category, they were getting
some treatment for necrotic enteritis,"
Broussard explained.
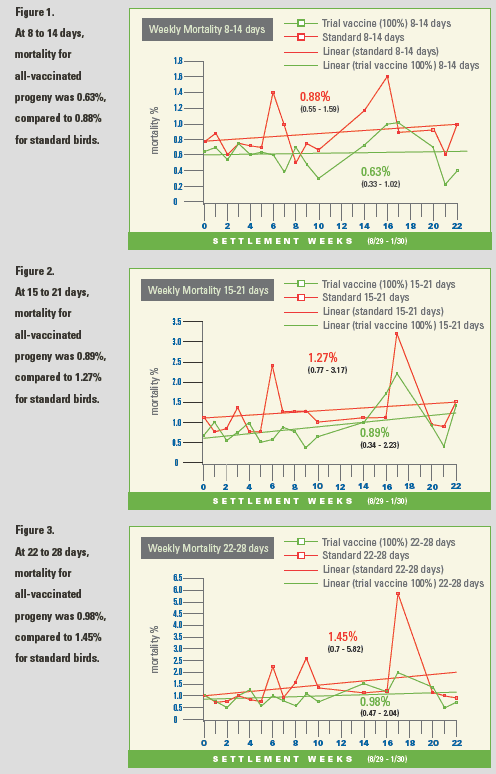
As to results of the trial, Broussard
first compared the performance of all vaccinated
progeny versus standard birds
early in the trial, at days 8 to 14 (Figure 1).
"Even at this point, we were already seeing
a difference of approximately a 0.25% in
mortality—0.63% for the all-vaccinated
progeny and 0.88% for the standard birds,"
he said.
During the next evaluation period, 15-21
days, when mortality tends to be on the
rise, mortality rates were .89% for the
all-vaccinated progeny and 1.27% for
the controls (Figure 2).
Historically, for companies raising broilers,
the next period of growth—days 22-28—would be a high-mortality time for NE, and
that was indeed the case for the company
that hosted the trial. Even during this difficult
time, however, vaccinated progeny
continued to do well, with 47% reduced
mortality, .98% versus 1.45% in standard
birds, Broussard said (Figure 3).
What particularly caught the eye of the
host company, Broussard said, was when
the researchers carrying out the study broke out mortality data for only the colder,
wetter months.
"When we looked at only that particular
time, when the company had told us that
they—like a lot of companies—had
experienced their main NE challenge, we
found that progeny from the vaccinated
hens had 72% less chance of mortality
than progeny in the standard group,"
Broussard said, adding that the difference
takes into account that some of the birds
in the standard group had come from
vaccinated hens.
Broussard said the host company was
impressed by the results of the trials. "So
much so that they recently decided to
vaccinate all their breeder flocks with the
NE toxoid," he reported.
Editor's note: The poultry company involved
with the NE toxoid trial agreed to share this
data on the condition that the company's
name and location not be reported.
Spring 2008
Regresar a North American Edition (#1)






 © 2000 - 2021. Global Ag MediaNinguna parte de este sitio puede ser reproducida sin previa autorización.
© 2000 - 2021. Global Ag MediaNinguna parte de este sitio puede ser reproducida sin previa autorización.