Working UndercoverSubclinical necrotic enteritis often goes undetected, slowly erodes profits
The disease is subclinical necrotic enteritis (NE), caused by the pathogen Clostridium perfringens. Poultry veterinarians familiar with subclinical NE say the industry needs to intensify efforts to detect and manage the disease before it erodes performance and profits. “It’s a big problem,” even though no one really knows the actual incidence because it is not easily detected or diagnosed, says Dr. Steve Davis, president and chief executive officer of Colorado Quality Research, Wellington. “The effect is subtle and additive. Performance deteriorates from flock to flock, especially if you aren’t cleaning out your houses. The clostridium in the environment increases over time,” he says. Conventional flocks at risk Subclinical NE is most likely to be seen in standard broilers that are receiving ionophores for coccidiosis control but not antibiotic growth promoters (AGPs), a pattern becoming more typical in the US poultry industry, Davis says. “Here at Colorado Quality Research, we saw the direction the industry was heading about 4 years ago and knew that necrotic enteritis was going to be a hot research topic,” he adds. “That’s why we developed and fine-tuned a live NE challenge model and, to date, have conducted over 30 NE studies.” AGPs, Davis explains, have an inadvertent side benefit. They not only promote growth, they control clostridium. Overuse of ionophore anticoccidials, coupled with extended withdrawal periods, has resulted in resistant coccidia and late cycling of the coccidial challenge, which results in poor gut health, setting the stage for development of NE. In contrast to subclinical NE, fullblown clinical outbreaks of NE are easily recognized and usually treated due to high mortality. They are more likely to occur in antibiotic-free birds and in birds receiving chemical anticoccidials or coccidiosis vaccines, which can do an excellent job controlling coccidiosis but have no secondary antibiotic effect against NE, says Davis, who has experience as a live production poultry veterinarian. How C. perfringens affects a flock might also differ with the isolate. Some C. perfringens isolates tend to cause rapid illness and mortality with less impact on the performance of surviving broilers, while others rarely cause mortality but significantly decrease growth and feed conversion, he says. Missed diagnosis “Producers tend to miss subclinical NE in part because they haven’t had it before,” he says. In other cases, there’s a gradual drop in feed efficiency and poor weight gain that goes unnoticed. The problems may have gone on for so long that producers think it’s normal. There may be liver lesions that result in condemnation at processing. Sometimes, subclinical NE isn’t recognized until a change is made in the health program and they see improved performance. Davis’ work has led him to believe that subclinical NE affects not only the gut, but also the joints in the form of synovitis and femoral head necrosis. “Generally, this problem is chalked up to an Escherichia coli infection, but if you culture the lesions anaerobically, I think you’d find clostridium is a much bigger issue because it allows bacteria to become systemic and it ends up in the joints,” he says, noting that he presented the research supporting this theory at the 2006 American Veterinary Medical Association annual meeting in July. Dr. Scott Gustin, director of veterinary services for North America and Asia at Cobb-Vantress, the Arkansasbased poultry research and development company that sells broiler breeders, has similar comments about subclinical NE. “Unless you’re looking for it during routine posting sessions, you’ll miss it,” he says. Does he believe that subclinical NE will increase as less AGPs are used? “Absolutely.” There is still a lot unknown about subclinical NE, Gustin says. “With the declining use of in-feed antibiotics and rise in intestinal disease, we are realizing that gut health is an incredibly complex science. Diseases or syndromes such as ‘dysbacteriosis’ and subclinical NE are perfect examples of newly evolving diseases we don’t understand and control optimally.” He’s seen enough subclinical NE, however, to conclude that “it’s probably much more of a significant issue than we realize and is probably much more widespread than clinical NE.” Cost of subclinical NE An oft-quoted figure on the cost of subclinical NE is 5 cents per bird. Gustin says that “Subclinical necrotic enteritis can result in a 5- to 10-point increase in feed conversion in controlled trials we have funded. It can be very damaging to economic returns across an operation due to that lost feed conversion ratio.”
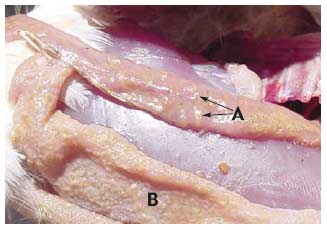
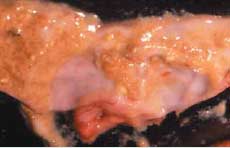
“You may have a large number of flocks affected before you realize you are losing feed efficiency and growth unless you conduct regular weekly posting sessions, which most producers do not,” he says. Hofacre likewise expects to see more acute and subclinical NE as the industry backs off the use of in-feed antibiotics. He cites the experience in Europe, where the poultry industry has seen a huge surge of NE in broilers since AGPs were banned by regulators. “In the US, the reduction in AGPs is being driven by consumers, not by regulators as it is in Europe. I don’t know that we’ll go to zero AGPs, but I think the US poultry industry will go pretty low. Consumers don’t want AGPs used,” he says. Causes of NE C. perfringens is a gram-positive, anaerobic bacterium that is normally found in the environment and gut of healthy birds. Although NE is caused by C. perfringens, not all C. perfringens causes NE, Hofacre points out. A toxic strain of the pathogen must be present. Most occurrences of NE are thought to be caused by the alpha (á-toxin) produced by C. perfringens. A toxic strain of C. perfringens takes hold when the bird’s intestines are damaged by other diseases, particularly coccidiosis, he says. “With intestinal damage, the normal balance of bacteria is disrupted and mucus production increases. That’s how the intestines respond, by coating their surface. But mucus also provides a food source for C. perfringens. The toxin utilizes mucus and further damages the intestines, and you get a damaging cycle,” Hofacre says. Recent evidence about the impact of coccidiosis on the development of NE comes from a study that was presented in July 2006 at the annual conference of the American Association of Avian Pathologists. It demonstrates that both coccidial pathogens Eimeria acervulina and Eimeria maxima cause enough intestinal damage to allow C. perfringens to proliferate. In the study, conducted by Dr. Greg Mathis of Southern Poultry Research, Athens, Georgia with co-investigator Hofacre, birds were inoculated with the Eimeria species then challenged with C. perfringens. Those with Eimeria infections were more susceptible to the development of NE compared to birds without coccidial infections. Both Eimeria species were associated with an increased risk for NE, but the risk was greatest with E. maxima. Davis says that another contributor to NE is animal byproducts. “We have conducted research showing there are literally tens of thousands of clostridium spores per gram in some animal byproducts found in blends. In one study we did, fish meal in the feed was heavily contaminated with C. perfringens. We’ve also found the pathogen in bone meal. So, in some cases, flocks are getting clostridium from their food.” There is a wrongful perception that chemical anticoccidials and coccidiosis vaccination cause NE, he says. “In fact, the opposite is true. These products in no way cause NE. They just don’t have direct efficacy against bacterium. There’s no secondary antibiotic effect,” he says. A clinical impression No tests for C. perfringens are currently available, but researchers are hoping to change that. Investigators in Europe, for instance, have been working on a blood test that would show antibodies to toxic C. perfringens. Hofacre cautions that there is also no way to predict which farms are at increased risk for NE based on litter samples. “Culturing C. perfringens from the litter is an exercise in futility,” he explains, because there is no way to tell if any clostridium that is isolated has the gene needed to produce the toxin that results in NE. In other words, “You can have a high level of clostridium in the environment, but that doesn’t prove it is causing disease,” he says. The lack of tests for identification of toxic C. perfringens means that diagnosis of subclinical NE depends on an astute clinician. “It’s a clinical impression among those experienced with the disease,” says Hofacre. “There’s a certain amount of enteritis, but it never gets severe enough to call it clinical NE. The term subclinical enteritis really reflects the severity of NE.” Recently he visited a farm with enteritis and liver lesions that appeared to be caused by NE. “This farm is on the tip of flipping over into acute NE, but I won’t allow birds to go untreated just to prove I’m right. I think this happens a lot.” It was a conventional flock and he suspects that their anticoccidial was beginning to lose efficacy and that too much coccidia was leaking through, Hofacre says. Says Gustin, “The diagnosis of subclinical NE relies heavily on the quality and frequency of technical service by those with the ability to diagnosis subclinical NE. Routine posting sessions conducted by individuals with special training in diagnosis of the disease are needed, and performance data must be correlated to necropsy findings, especially during times that coccidiosis programs are transitioning.” Davis adds, “To accurately diagnosis subclinical NE, I think it’s very important to get anaerobic cultures of very fresh, dead birds by euthanizing if necessary.” Once familiar with the disease and the intestinal lesions that characterize NE, says Hofacre, subclinical NE can be diagnosed upon necropsy. The small intestinal surface is covered with mucus and may be thick and rough. The liver may be firm and is a dark, mahogany color. Hofacre uses a scoring system with a range of zero to three for assessing necrotic enteritis, he says. Zero would be no intestinal lesions, one would be a mucus-covered intestine, characteristic of subclinical NE, while scores of two to three would indicate clinical NE because the intestines would be obviously diseased with bloody exudate in birds about to die or that died.
NE control strategies To best control both clinical and subclinical NE in flocks, especially for producers backing off antibiotic use, a combination approach will most likely be needed, starting with good coccidiosis control, say these experts. “One combination might be coccidiosis vaccination, natural products such as organic acids and NE vaccination,” says Davis, who believes that a key to any successful combination will involve immunity. “Consider dermatitis, which is a clostridial disease that occurs later in the life of birds, unlike NE, which occurs early in the bird’s life. I have never seen or met another poultry veterinarian who has seen a flock of broilers with NE that get dermatitis later, and the reason for that is immunity,” he says. In short, birds that survive NE build immunity against dermatitis. That observation indicates that vaccinating for NE could be helpful in flocks at risk. This would include not just antibiotic-free birds, but birds receiving chemicals or vaccines to prevent coccidiosis and birds on a rotation program during times they transition off ionophores, he says. A C. perfringens type A toxoid vaccine for NE has been developed by Schering-Plough Animal Health and, at this writing, is being used in the US with a conditional license granted last year by the USDA. It is administered to breeders, which convey passive immunity against NE to their broiler progeny. The vaccine has obvious benefits for antibiotic-free birds at risk for acute NE outbreaks and might also benefit producers still using AGPs by enabling them to lower the amount of antibiotic used, says Davis. When his firm vaccinated birds with the type A toxoid then challenged their progeny with clostridium, they performed just as well whether they received 25 grams/ton of bacitracin methylene disalicylate (BMD) or twice that amount. “There seemed to be some synergistic effect between the vaccine and BMD.” Gustin couldn’t comment specifically on the new vaccine but says, “If you look at many of the diseases we vaccinate for in poultry, it is obvious that we take advantage of passive immunity. We should do so for other diseases if we can do so effectively. “One would assume that if clinical necrotic enteritis can be prevented with a vaccine, the same mechanism could improve control of subclinical NE. Since subclinical NE is much more widespread than clinical NE, the benefits to conventional sectors would be there,” he says. Hofacre says, “When anticoccidials start to fail, a vaccine may have a role” and it may also have a role in birds receiving AGPs, since antibiotics are not always 100% effective against NE. Producers, however, will have to weigh the cost of the vaccine against other factors.” To improve NE control, he advises that producers “look at ways to maintain good gut flora and overall intestinal health, perhaps by feeding competitive exclusion products and organic acids. If a coccidiosis vaccine is used, make sure it’s managed well” and administered properly so that birds do not get overexposed to coccidia. Control of NE also relies on good basic management, these experts emphasize. Litter moisture, house temperature, the diet fed to flocks as well as bird density and other factors must be considered and will play an increasingly important role in the control of NE (see article, page 6).
Source: CocciForum Issue No.12, Schering-Plough Animal Health. |
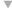




 © 2000 - 2021. Global Ag MediaNinguna parte de este sitio puede ser reproducida sin previa autorización.
© 2000 - 2021. Global Ag MediaNinguna parte de este sitio puede ser reproducida sin previa autorización.